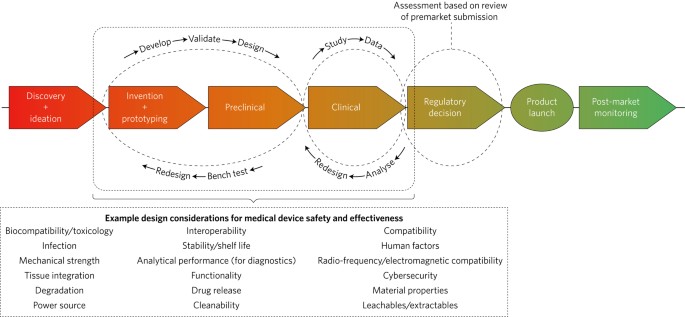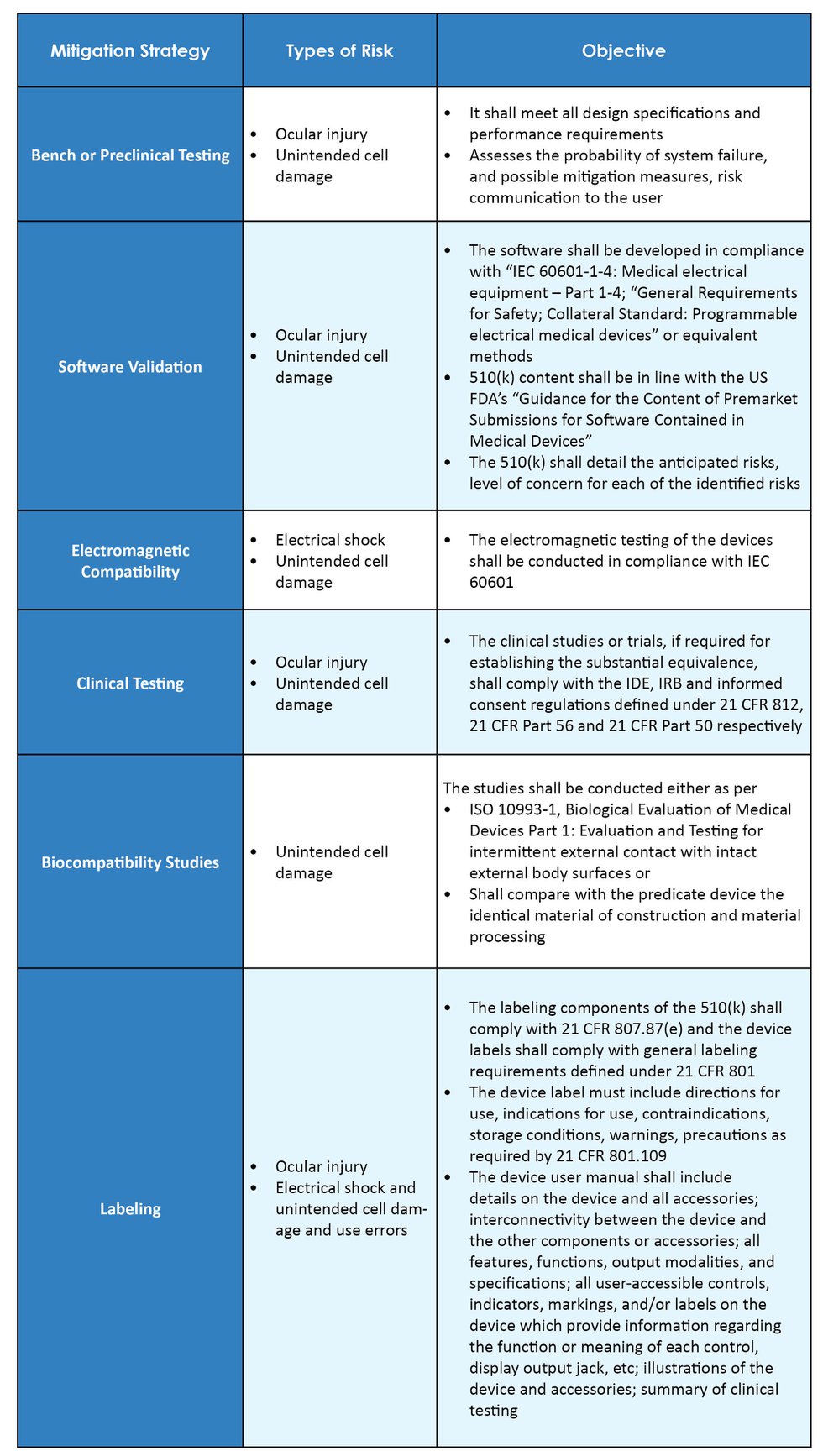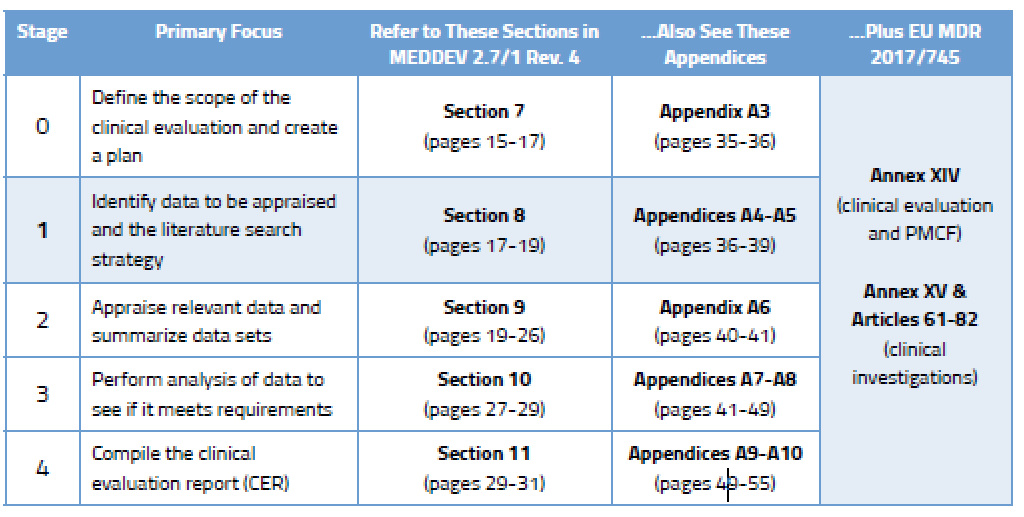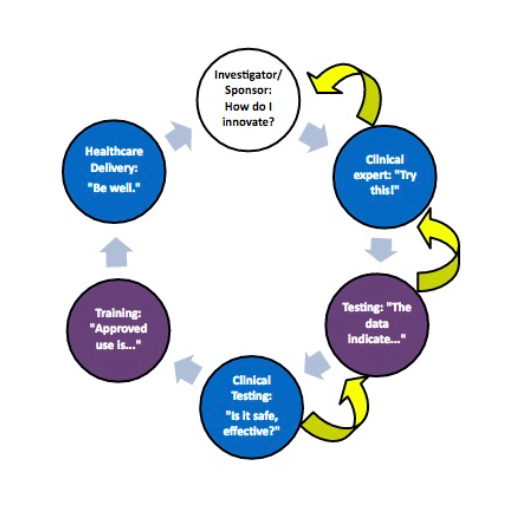
From Concept To Cure, How To Secure FDA Approvals on Preclinical Medical Device Testing. - GCMI Atlanta - Pre Clinical CRO

510k submission to the FDA (case study-part 1) Archives - Medical Device Academy Medical Device Academy

Argon Medical Devices, Inc. on LinkedIn: Bench Testing Comparative Data Relative To Drainage Catheter Performance :…