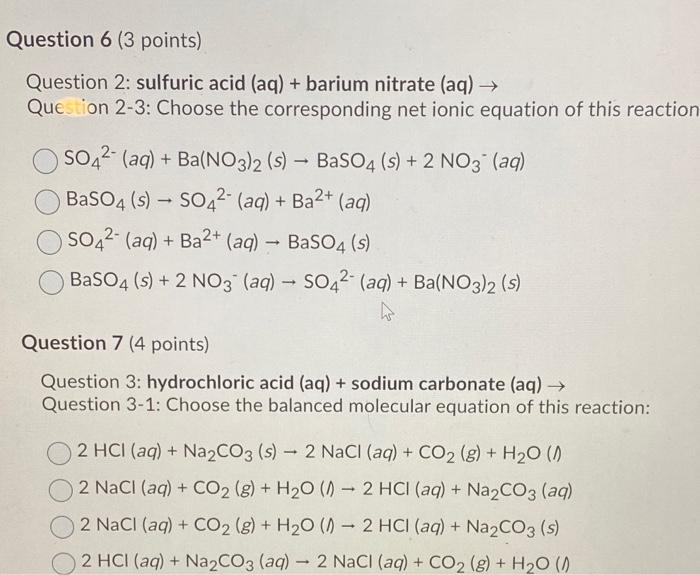
Minerals | Free Full-Text | Selective Fabrication of Barium Carbonate Nanoparticles in the Lumen of Halloysite Nanotubes

Procedure method making insoluble salt by precipitation reaction from two soluble compounds apparatus chemicals procedures equations use of barium sulfate meal gcse chemistry KS3 KS4 Science IGCSE O level revision notes
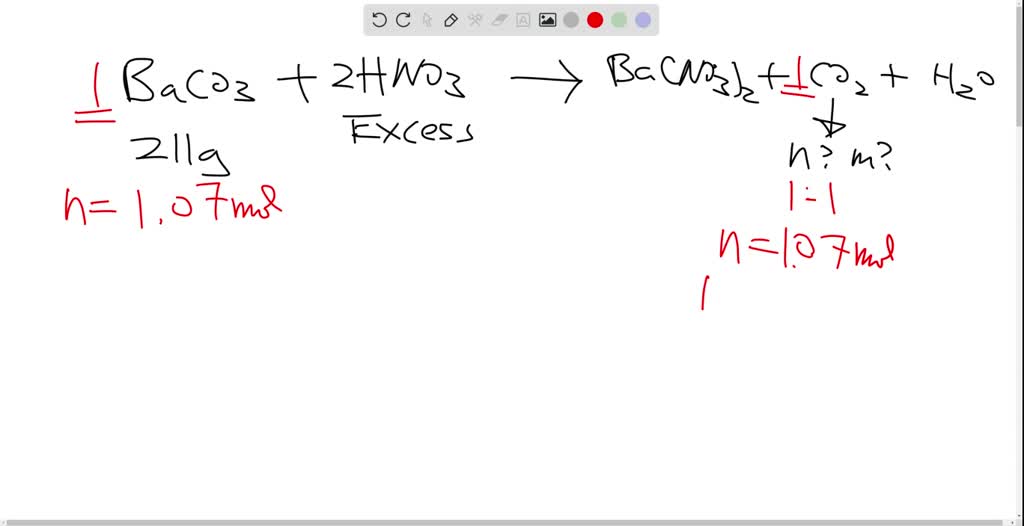
SOLVED:A 211 g sample of barium carbonate, BaCO 3, reacts with a solution of nitric acid to give barium nitrate, carbon dioxide and water. If the acid is present in excess, what
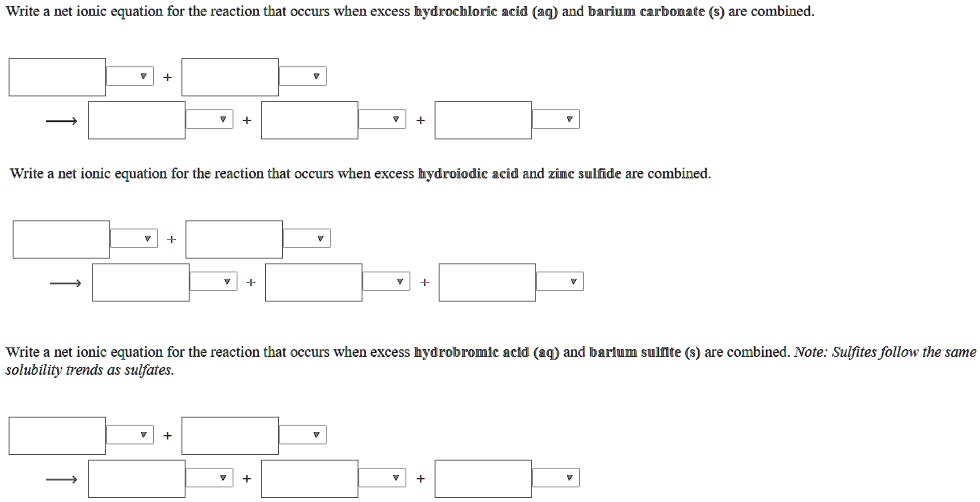
SOLVED: Write net ionic equation for the reaction that occurs when excess hydrochloric acid (aq) and barium carbonate are combined Write net ionic equation for the reaction that occurs when excess hydroiodic

Write a balanced chemical equation for the reaction of calcium carbonate and dil. hydrochloric acid.

OneClass: hydrochloric acid plus barium hydroxide yields barium chloride pluswaterWrite a balanced eq...