ASCO-GI 2023: Feasibility of circulating tumor DNA (ctDNA) to guide organ preservation in patients with node-negative rectal cancer undergoing neoadjuvant chemotherapy, excision, and observation in the phase II CCTG CO.28 trial.

trademark trader on Twitter: "FOUNDATIONONE TRACKER is being trademarked by Foundation Medicine, Inc. https://t.co/yjLY8VFLSJ #FOUNDATIONONETRACKER $FMI # FOUNDATIONONE #TRACKER https://t.co/R8MNxfo7qN" / Twitter

Foundation Medicine's ctDNA Monitoring Assay Granted FDA Breakthrough Device Designation | Clinical Lab Products

ASCO-GI 2023: Feasibility of circulating tumor DNA (ctDNA) to guide organ preservation in patients with node-negative rectal cancer undergoing neoadjuvant chemotherapy, excision, and observation in the phase II CCTG CO.28 trial.

Foundation Medicine's ctDNA Monitoring Assay Granted FDA Breakthrough Device Designation | Clinical Lab Products
Comprehensive Genomic Profiling (CGP)-Informed Personalized Molecular Residual Disease (MRD) Detection: An Exploratory Analysis
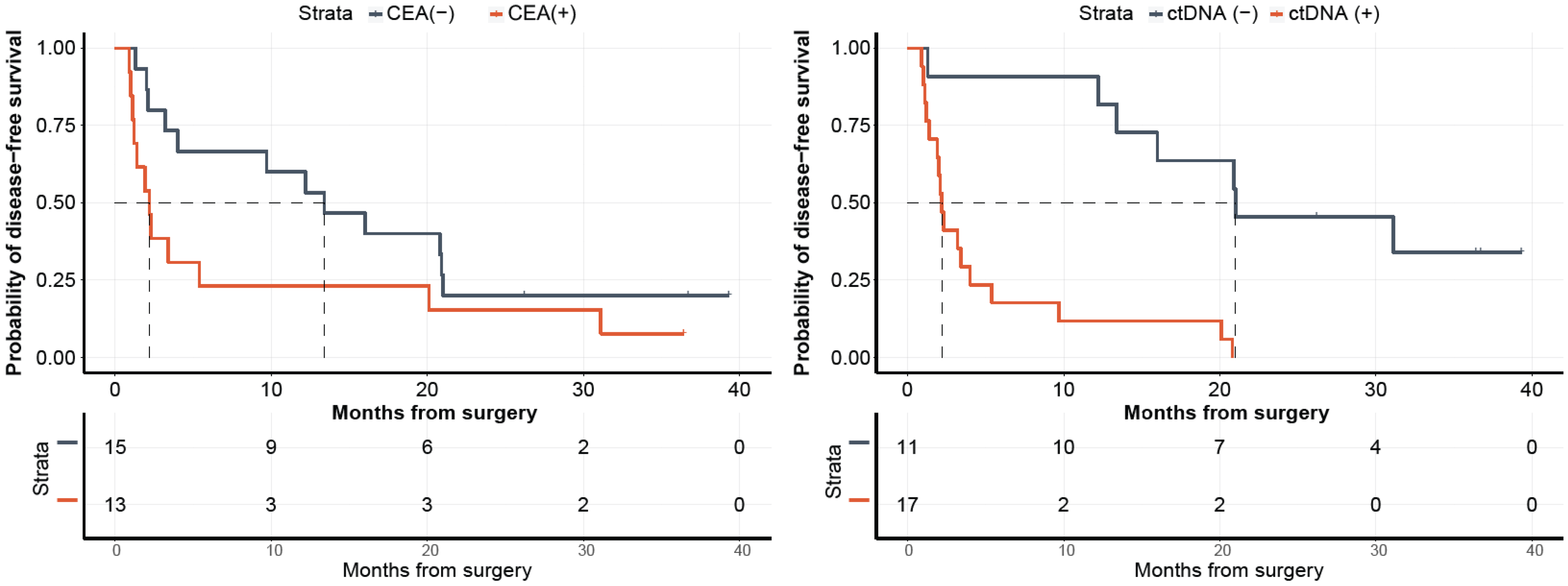
IJMS | Free Full-Text | Comprehensive Genomic Profiling (CGP)-Informed Personalized Molecular Residual Disease (MRD) Detection: An Exploratory Analysis from the PREDATOR Study of Metastatic Colorectal Cancer (mCRC) Patients Undergoing Surgical Resection

ASCO-GI 2023: Feasibility of circulating tumor DNA (ctDNA) to guide organ preservation in patients with node-negative rectal cancer undergoing neoadjuvant chemotherapy, excision, and observation in the phase II CCTG CO.28 trial.

Foundation Medicine and Natera Announce the Launch of FoundationOne®Tracker Personalized Circulating Tumor DNA Monitoring Assay for Investigational Use and Early Access Clinical Use | Business Wire

Dymeka Harrison sur LinkedIn : Foundation Medicine just launched FoundationOne Tracker in collaboration… | 86 commentaires