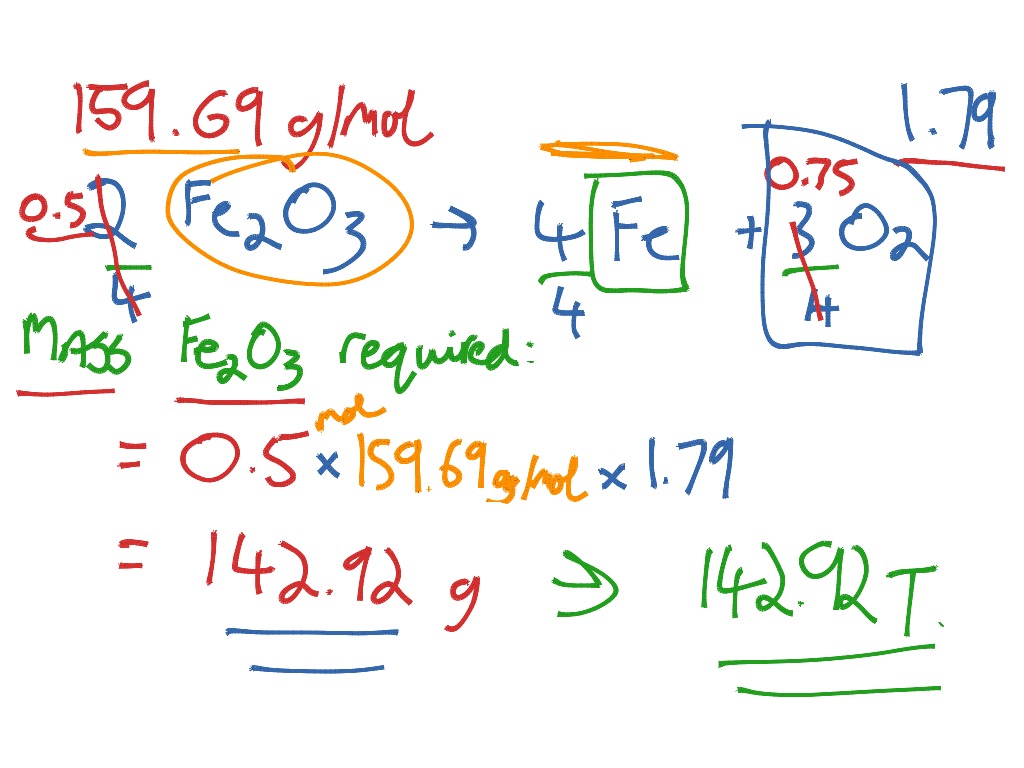
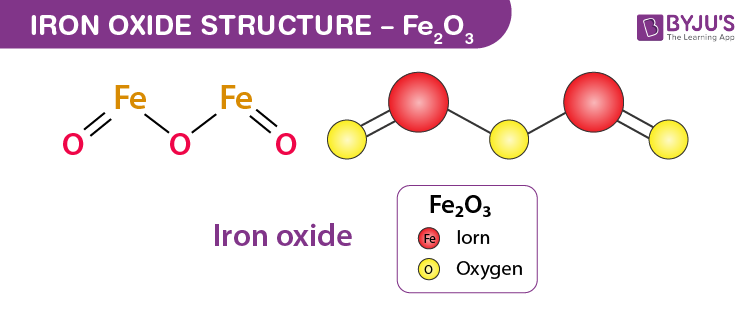
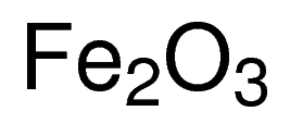SOLVED: Solid iron(III) oxide reacts with hydrogen gas to form solid iron and liquid water: Write the balanced chemical equation for the rcaction described. Phases are optional equation:

Iron precursor hydrated iron (III) oxide (FeO(OH)) placed on aluminum... | Download Scientific Diagram

Question Video: Identifying Iron Oxide Produced from the Reaction of Unknown Salt with an Alkali Solution | Nagwa