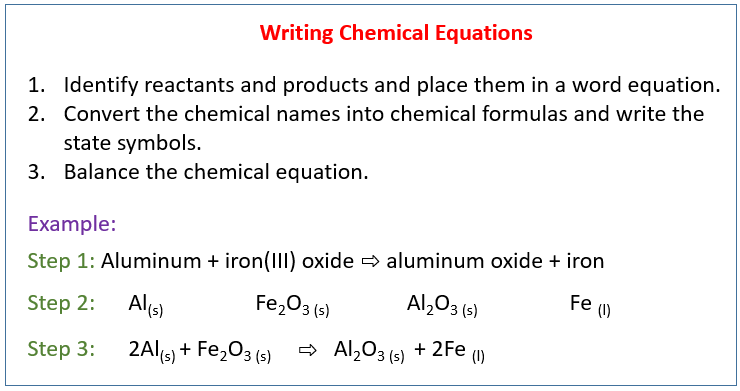
Chapter 8 Section 1 Chemical Equations reactants → products reactant- starting substance in a chemical reaction product- substance formed in a chemical. - ppt download

Lakhmir Singh Chemistry Class 10 Solutions For Chapter 1 Chemical Reactions And Equations - Free PDF

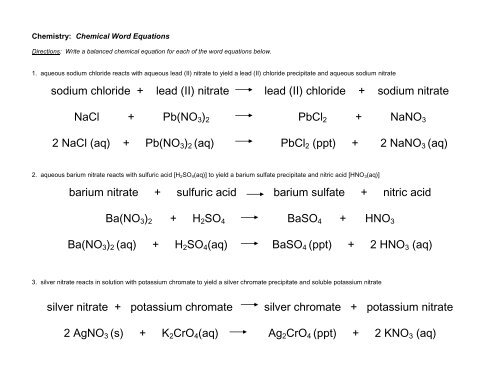
Word Equations Fill in the gaps in these word equations to make them complete. If there is no reaction write this in the gap. Sodium + water. - ppt download

Complete the following word equations and then a chemical equation for each reaction.a) Zinc + lead - Brainly.in

SOLVED: Complete the following word equations and then a chemical equation for each reaction.a) Zinc + lead nitrate solution =b) Iron + zinc sulphate solution - lead + copper nitrate solutionc) Magnesium +

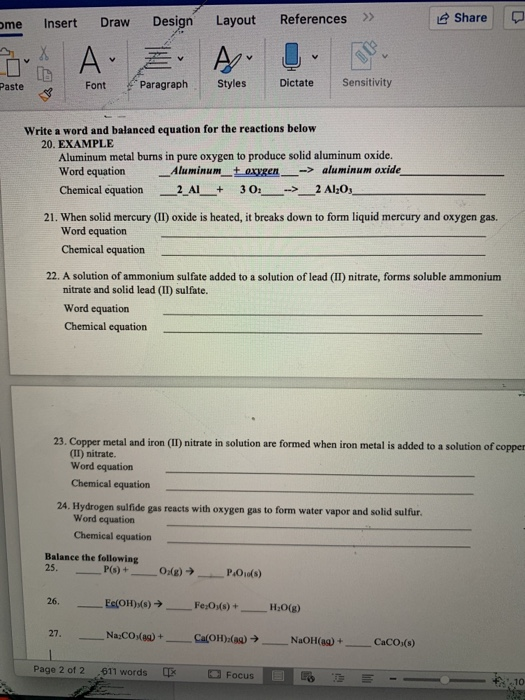
Practice For the following reactions, write: the word equation the skeleton equation (unbalanced chemical equation) the balanced equation 1. Potassium. - ppt download

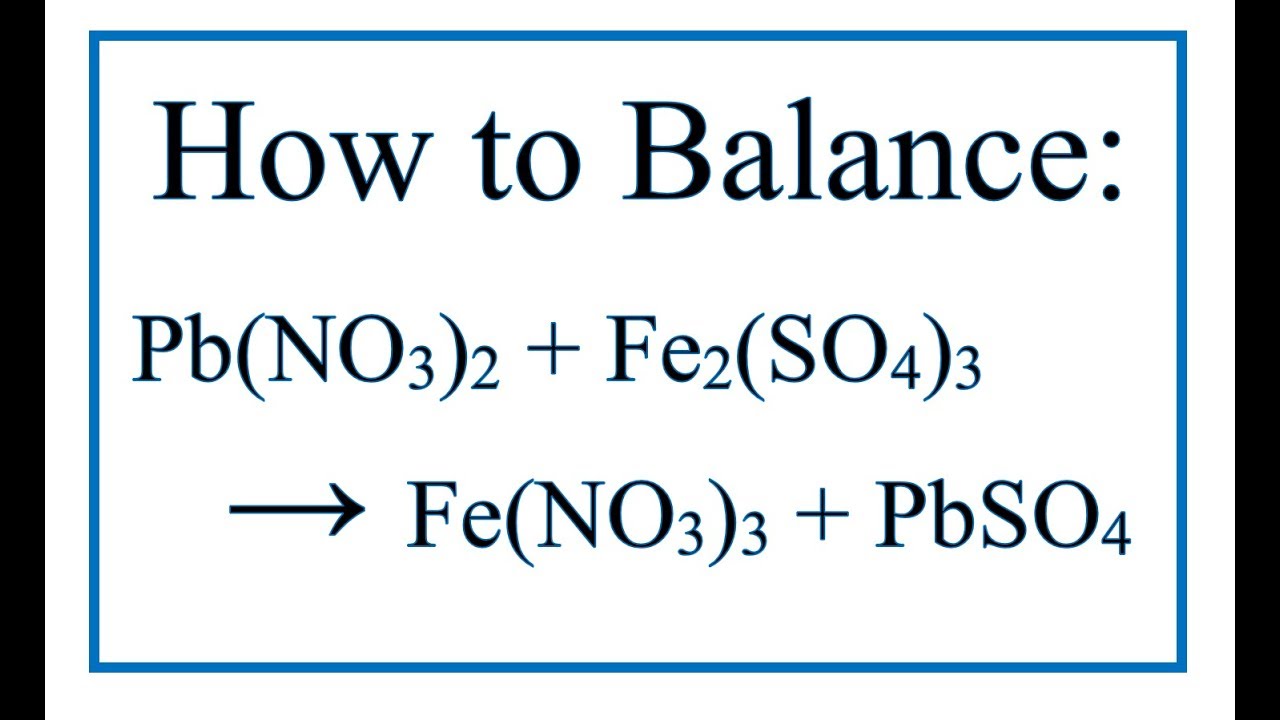
Write the balanced chemical equations for the following reactions.(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water(b) Zinc + Silver nitrate → Zinc nitrate + Silver(c) Aluminium + Copper chloride