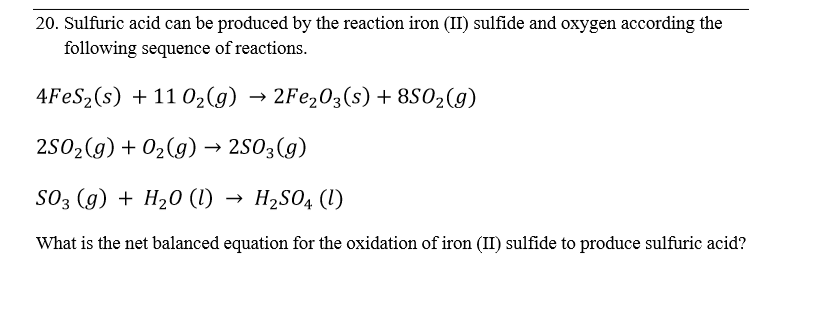
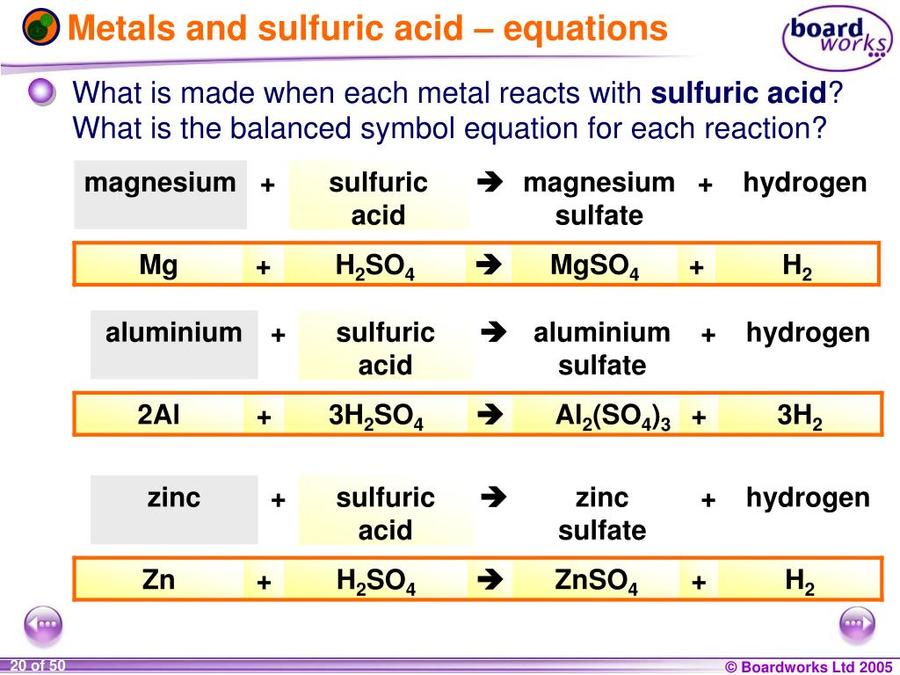
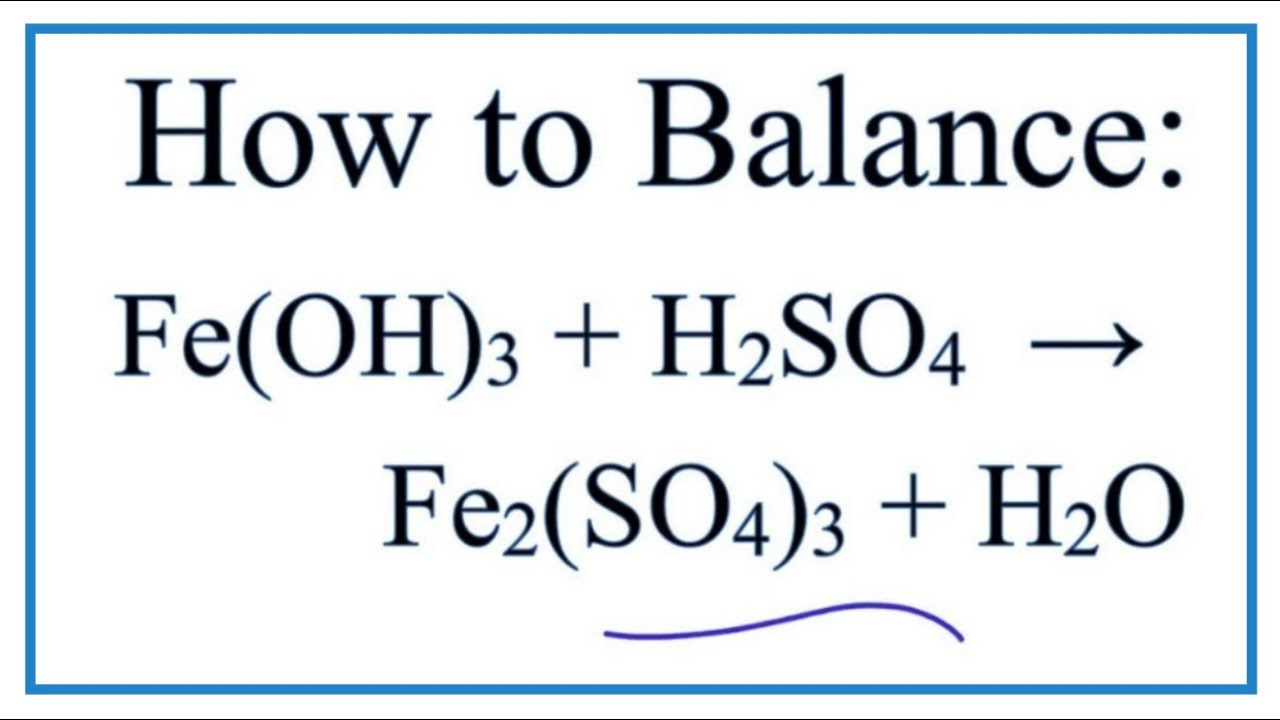
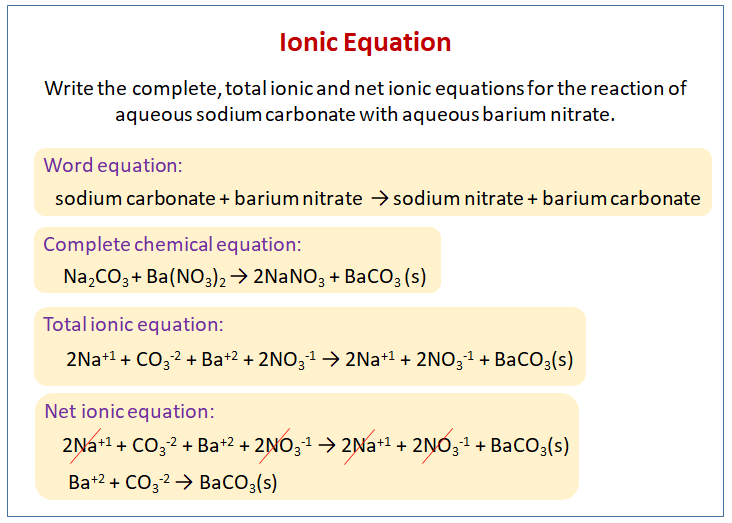
Write word equations and then balanced equations for the reaction taking place when:(a) Dilute sulphuric acid reacts with zinc granules.(b) Dilute hydrochloric acid reacts with magnesium ribbon.(c) Dilute sulphuric acid reacts with

How to Balance Fe + H2SO4 = FeSO4 + Fe2(SO4)3 + H2O + SO2 (Iron + Concentrated Sulfuric acid) - YouTube
an alloy of iron and carbon was treated with sulphuric acid in which only iron react . if a sample of alloy weighing 140gm gave 6gm of hydrogen . what is the
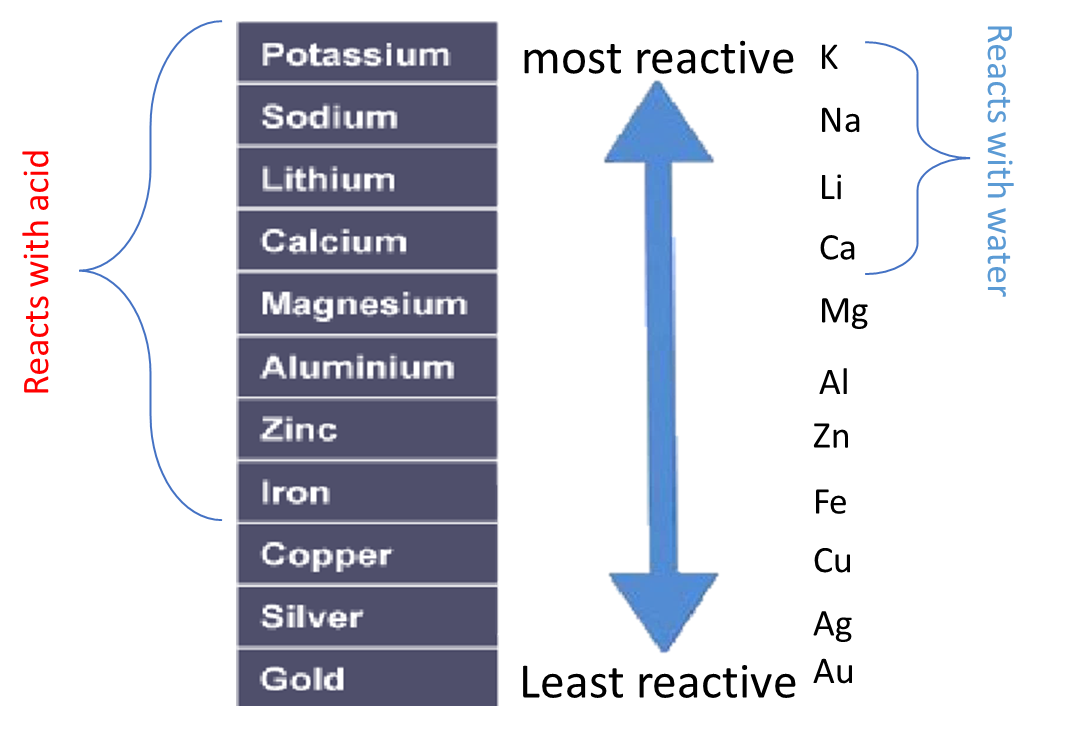
2:15 understand how metals can be arranged in a reactivity series based on their reactions with: water and dilute hydrochloric or sulfuric acid - TutorMyself Chemistry

Question Video: Identifying the Balanced Symbolic Equation for the Reaction of Iron Metal with Concentrated Sulfuric Acid | Nagwa
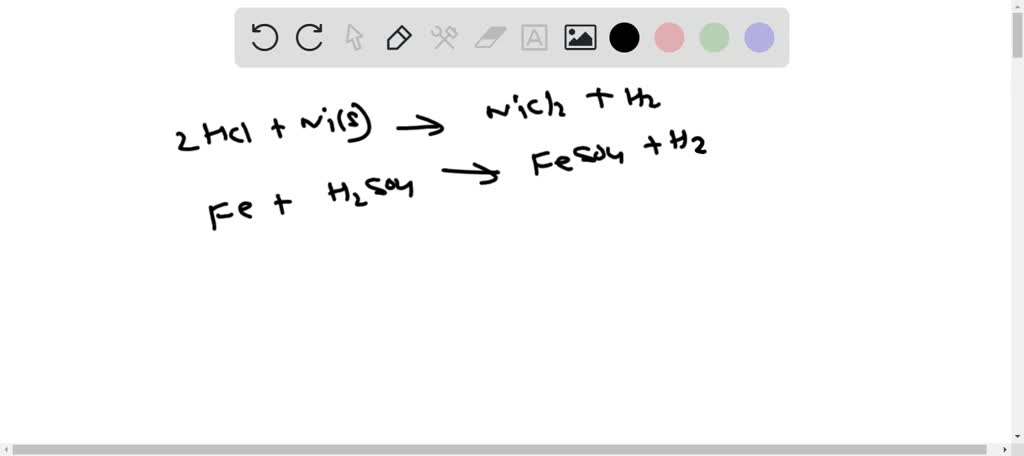
SOLVED: Write balanced molecular and net ionic equations for the reactions of (a) hydrochloric acid with nickel, (b) dilute sulfuric acid with iron, (c) hydrobromic acid with magnesium, (d) acetic acid, CH3COOH,