Three Things You Should Know about Medical Device Regulations in Russia, August 2015 | Medical Device Regulations in Russia and Eurasian Union
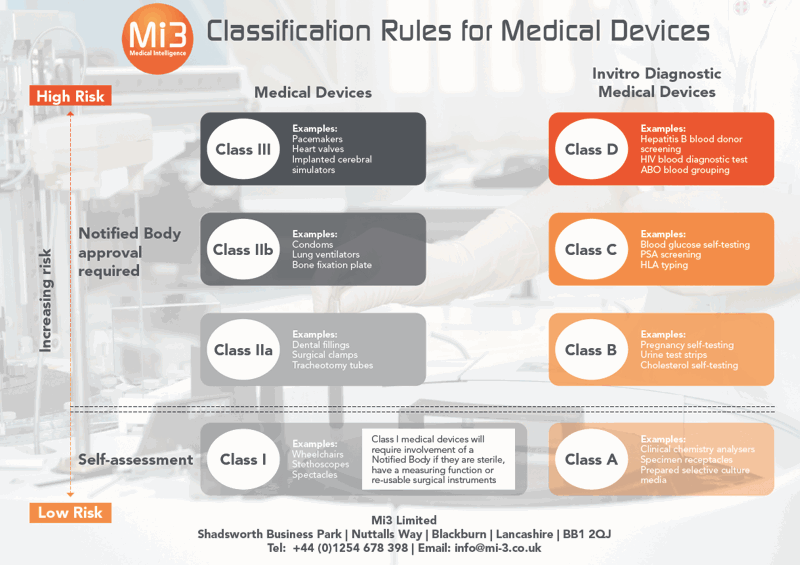
Introduction to Medical Devices and their regulatory framework: 1. Classification of Medical Devices and IVDs

How to create an aligned global co-development strategy for drug with device component combination products - Voisin Consulting Life Sciences






.webp)