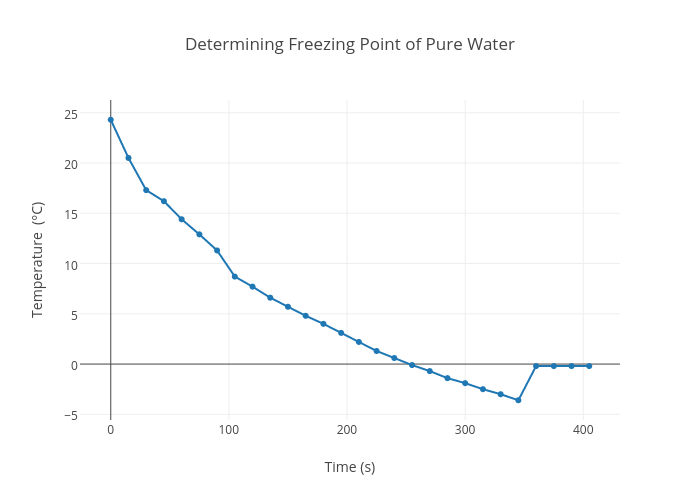
2 Typical freezing profile of frozen pure water and food systems. The... | Download Scientific Diagram

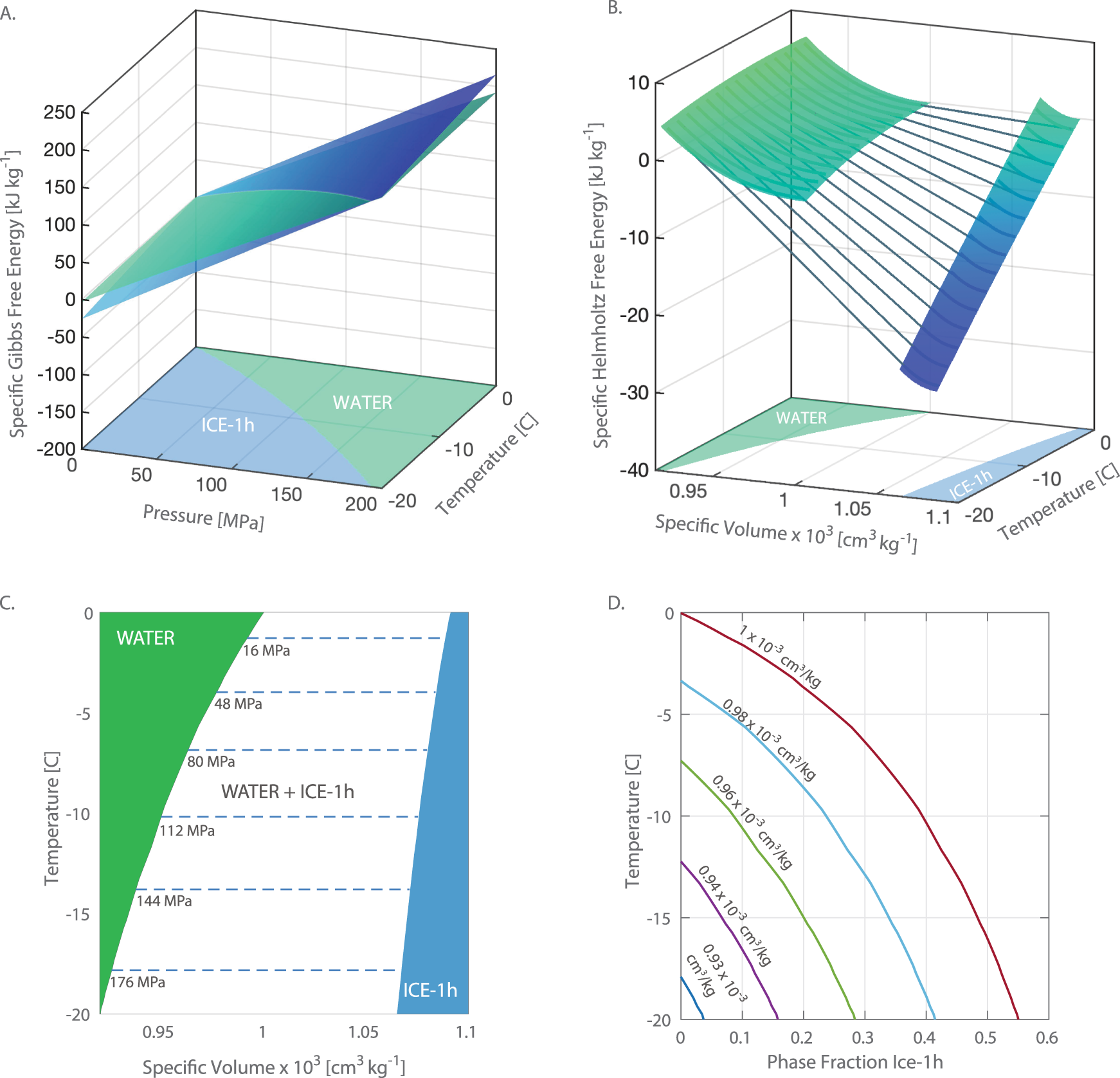
Freezing curve for pure water and various soil textures, according to... | Download Scientific Diagram

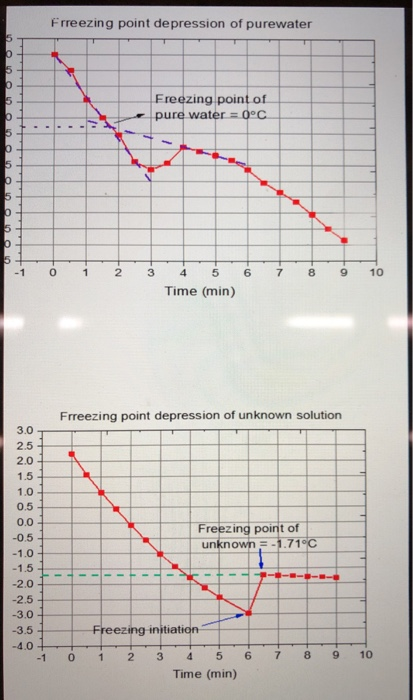
Purified water is able to be cooled below its freezing point but will immediately freeze when agitated. : r/Damnthatsinteresting

A 4% solution (w/w) of sucrose (M 342 g `mol^(-1)`) in water has a freezing point of 271.15K - YouTube
SOLVED: The freezing point of pure water is 0.0°C. How many grams of ethylene glycol (C2H6O2) must be mixed in 100.0 g of water to lower the freezing point of the solution

Pure water freezes at 273 K and 1 bar. The addition of 34.5 g of ethanol to 500 g of water changes the - Brainly.in


:max_bytes(150000):strip_icc()/blocks-of-melting-ice-200025919-001-586a81f93df78ce2c33aa8f9.jpg)