
Carbon monoxide was first prepared by Lasson in 1776,by heating zinc oxide with wood charcoal.It was mistaken for hydrogen, because it burnt with a pale. - ppt download

Megan Logue. Is a.1 M or a 1 M solution of sodium hydroxide more effective at extracting and absorbing carbon dioxide from air? Will carbon dioxide. - ppt download

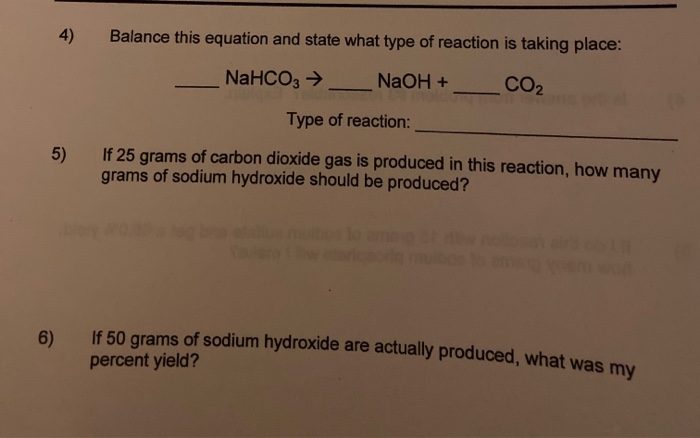
SOLVED:Sodium hydroxide reacts with carbon dioxide as follows: 2 NaOH(s)+CO2(g) ⟶Na2 CO3(s)+H2 O(l) Which is the limiting reactant when 1.85 mol NaOH and 1.00 mol CO2 are allowed to react? How many

Question Video: Deducing the Balanced Chemical Equation for the Reaction between Hot Sodium Hydroxide and Carbon Dioxide | Nagwa

Carbon Dioxide Capture from Atmospheric Air Using Sodium Hydroxide Spray | Environmental Science & Technology
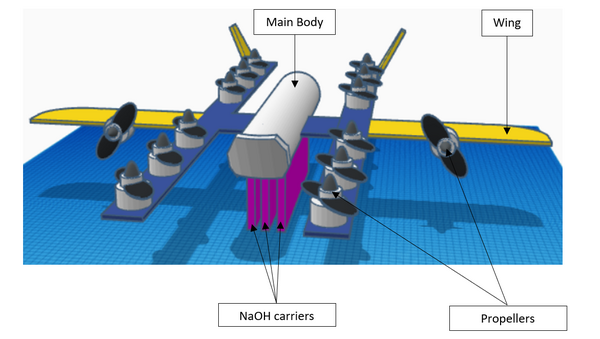
Use of drone with sodium hydroxide carriers to absorb carbon dioxide from ambient air | Journal of Emerging Investigators

Carbon Dioxide Capture from Atmospheric Air Using Sodium Hydroxide Spray | Environmental Science & Technology

Experimental study on capture of carbon dioxide and production of sodium bicarbonate from sodium hydroxide

Low-energy sodium hydroxide recovery for CO2 capture from atmospheric air—Thermodynamic analysis - ScienceDirect
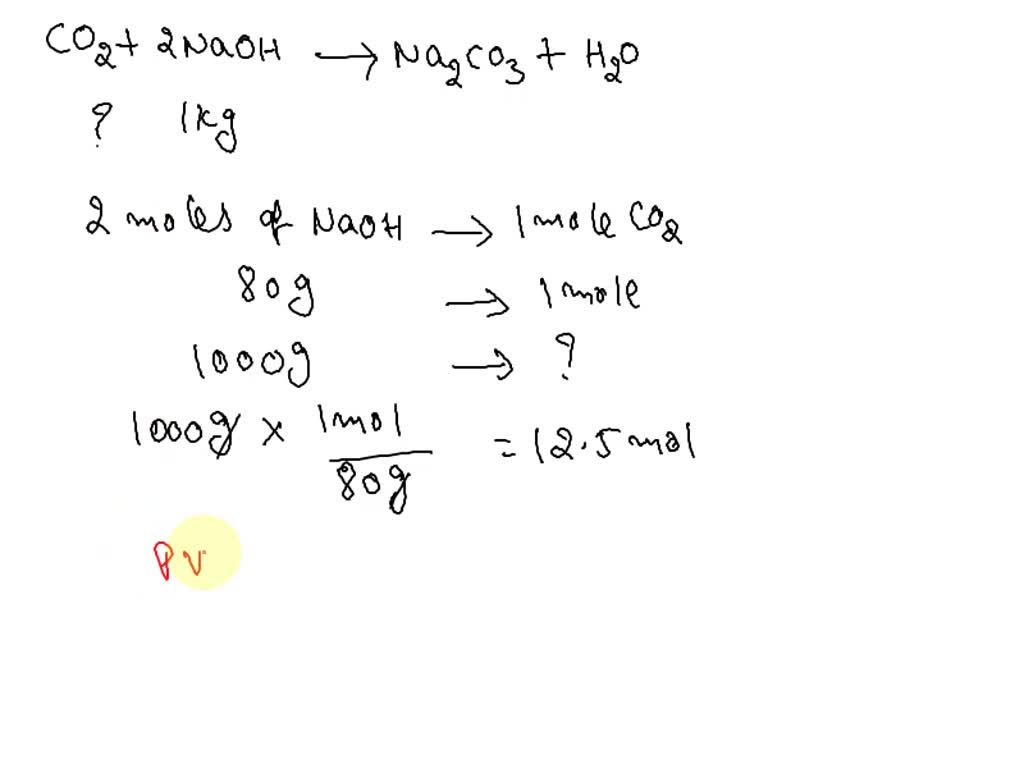



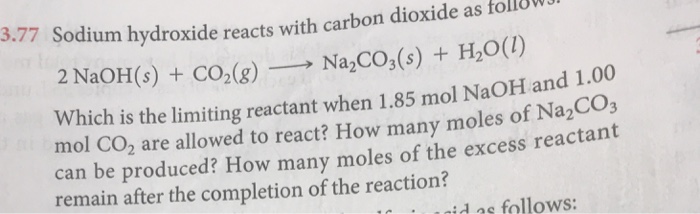
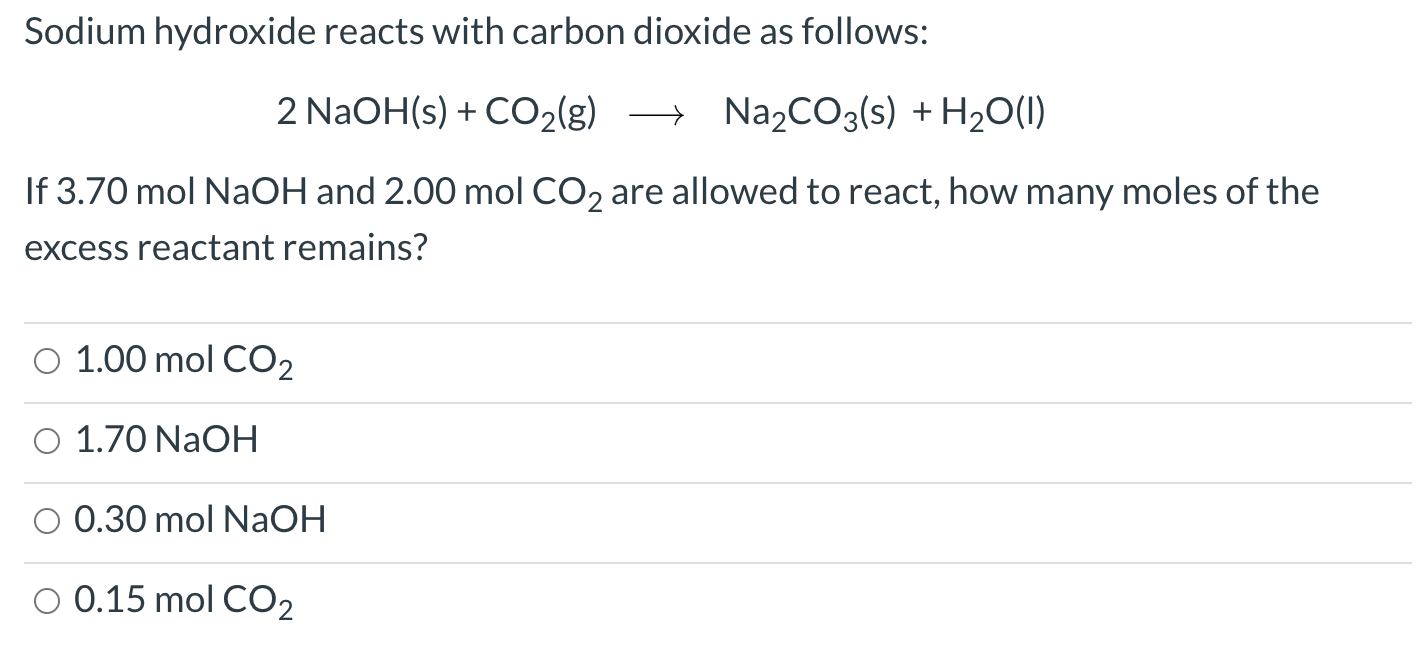



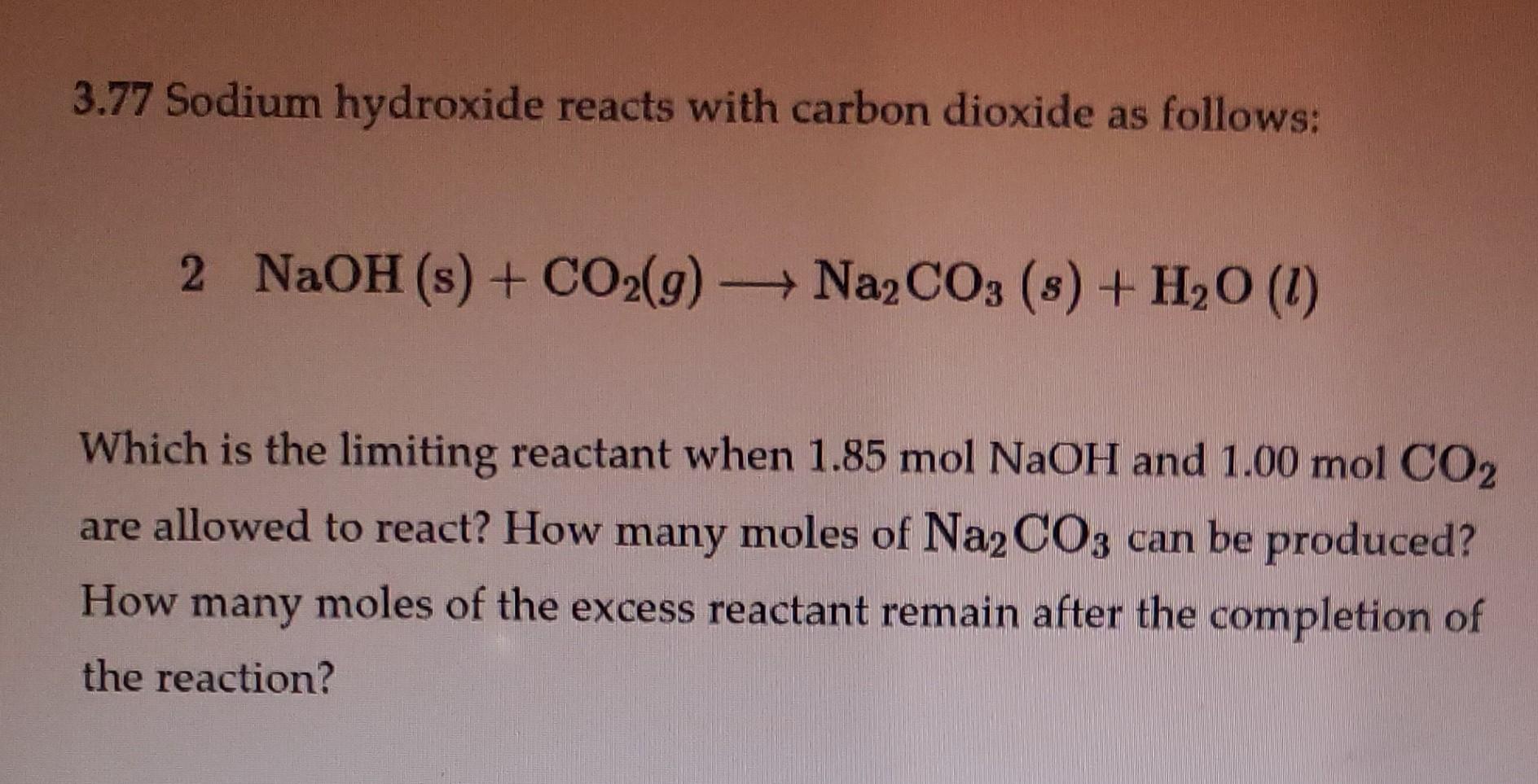






![PDF] Carbon Dioxide Capture from Atmospheric Air Using Sodium Hydroxide Spray | Semantic Scholar PDF] Carbon Dioxide Capture from Atmospheric Air Using Sodium Hydroxide Spray | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4811d2235d1d7b89e3e440e4d0ee950050f4714f/3-Figure1-1.png)
